Sign up for free
for our newsletter!
for our newsletter!

Loading...
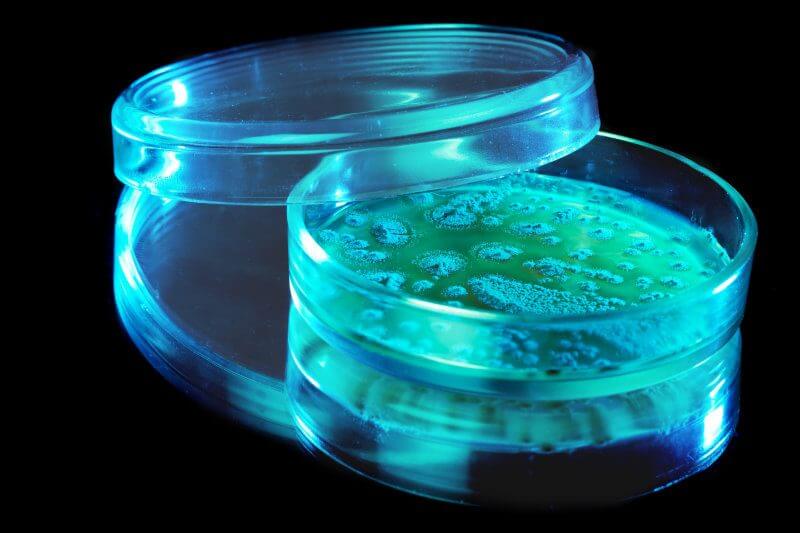
For years we are working successfully with the so-called small Molecules, R & D molecules such as alpha-Amanitin, CAS No.: 23109-05-9 – Ovalicin, CAS No.: 19683-98-8 – Geldanamycin, CAS No.: 30562 -34-6 – or APIs like Sirolimus / Rapamycin, CAS No .: 53123-88-9.
With all these products Cfm started in mg quantities. Now many of them are sold thanks to the help of our manufacturers and, of course, customers in kg quantities. Constant optimization of the fermentation processes, adaptation to new regulatory requirements and investments in production and marketing are necessary in order to be successful in the market.
You also have an interesting molecule with a promising application that you would like to have manufactured, but you lack a reliable manufacturer, or the previous quantities are too small for the other producers?
Ask us! We cooperate on a worldwide basis with qualified, reliable and long-term partners, which implement your project exactly according to your wishes and ideas.
Direct contact with our manufacturer is standard. On basis of this open and trusting cooperation, later upscaling projects, communicated in time, are easier to implement. We offer big corporation expertise in quality and our quality management system, BUT quick decision-making in all other aspects. Contact us!


Sourcing substances is always an exciting challenge. Even if product lists are very extensive, in our case we have more than 890 substances of fermentative origin listed, there is not exactly the substance you need.At this point the product sourcing of Cfm Oskar Tropitzsch GmbH starts. Often, the producing strains such as Aspergillus flavus or Phomopsis leptostromiformis, to name just two examples, are known. The desired substance can be produced easily by one of our partners in the worldwide network of cfm in the nonGMP or cGMP range.If the strain is not known, our partners rely on many years of knowledge and extensive core collections and screen for the one strain that produces your substance. The goal is always to realize your project.As an additional service, we check whether there are any restrictions (customs, regulations or legal ones (Nagoja Protocol)) that preclude their use in your project.The trusting exchange with our partners and the regular participation in fairs, congresses, symposia and exhibitions enable us to be always up-to-date with the latest trends in this field.Have we piqued your interest? Then just get in contact with us for further details, like to discuss in person.
All our partners share a great passion for their respective field of expertise. The network partners of Cfm Oskar Tropitzsch GmbH in the product segment “Fermentation/small molecules” regularly participate in expeditions, in order to constantly expand their core collection. Sponges, fungi, etc. are screened for new substances.In this way, the collections of our partners and thus possible solutions for your project are constantly expanding. We received the order for a substance from our portfolio and were confident that this would be an easy mission. However, shortly after we commissioned the manufacture of this substance, we received the news that the original strain was no longer producing, although it had always done so in recent years. Due to the extensive collection and the tireless efforts of our supplier, he found an alternative strain, which then produced the desired product and thus we were able to deliver to our customers on time.

Mycotoxins are the mold toxins, which are formed in unfavorable weather conditions or during storage in and on the plants. Mycotoxins can cause serious poisoning or even cancer. Particularly affected products are e.g. corn, cereals, milk and subsequently the processed foods such as the Parmesan cheese.In order to make life easier for food testing laboratories and to enable even better measurement results, we have expanded our portfolio with isotope-labeled mycotoxin reference standards. Either these deuterated or C13 labeled compounds allow for even more accurate and therefore safer analysis and thus more security for consumers.At the mycotoxin conference, which is also supported by our Cfm Oskar Tropitzsch GmbH, we refresh our knowledge to be always up to date with the latest scientific standards. Close contact with the leading European universities in this field helps us to react quickly to changing requirements.
Active ingredients, among others of microbial origin for the pharmaceutical field of application are subject to the highest requirements during import to the European Union. Responsible for this is §72a of the Medicines Act (AMG), which regulates the importation within the meaning of the AMG in the transfer of products from third countries to the European Union.Certain drugs and active substances can only be imported in accordance with §72a AMG if certain quality certificates are available from an authority of the country of manufacture (Written Confirmation). The situation is even more serious if there are no accepted basic rules between the country of the producer and the EU. Then the import can only be made by a passed inspection of the competent monitoring authorities directly at the manufacturer. For this scenario, we offer our extensive expertise and support. Transport processing, import, release analysis by qualified laboratories are included in this process.Furthermore, we qualify all our manufacturers and customers in the field of active ingredients through our GDP quality management system. Quality Assurance agreements are signed with the manufacturer prior to each purchase to provide the highest level of legal certainty to all parties involved in the process.
