
Element of the month April: Barium
The alkaline earth metal barium was first identified in 1774 by the pharmacist and chemist Carl Wilhelm Scheele, who contributed to the discovery of several elements, especially oxygen.
In its elemental state, barium appears silvery-white and shiny metallic. Because of its high reactivity, barium does not occur elementally in nature. It is the 14th most common element in the earth’s crust.
Use – from rat poison to pyrotechnics
Elemental barium is not used often, but for example as an additive for metal alloys and as a component of high-temperature superconductors, of vacuum tubes or of nickel-barium spark plugs.
Despite its toxicity, there are the following examples of its use:
• Barium carbonate is an effective rat poison.
• Barium oxide is a component of special glasses with a higher refractive index
• Barium nitrate and barium chlorate are considered oxidizing and turn flames green, which is why they are used in pyrotechnics.
• Barium chloride is used for hardening steel
Barium sulfate (baryte) is completely harmless due to its low solubility (10-5 moles per liter) and is therefore used as:
• “permanent white” for paints
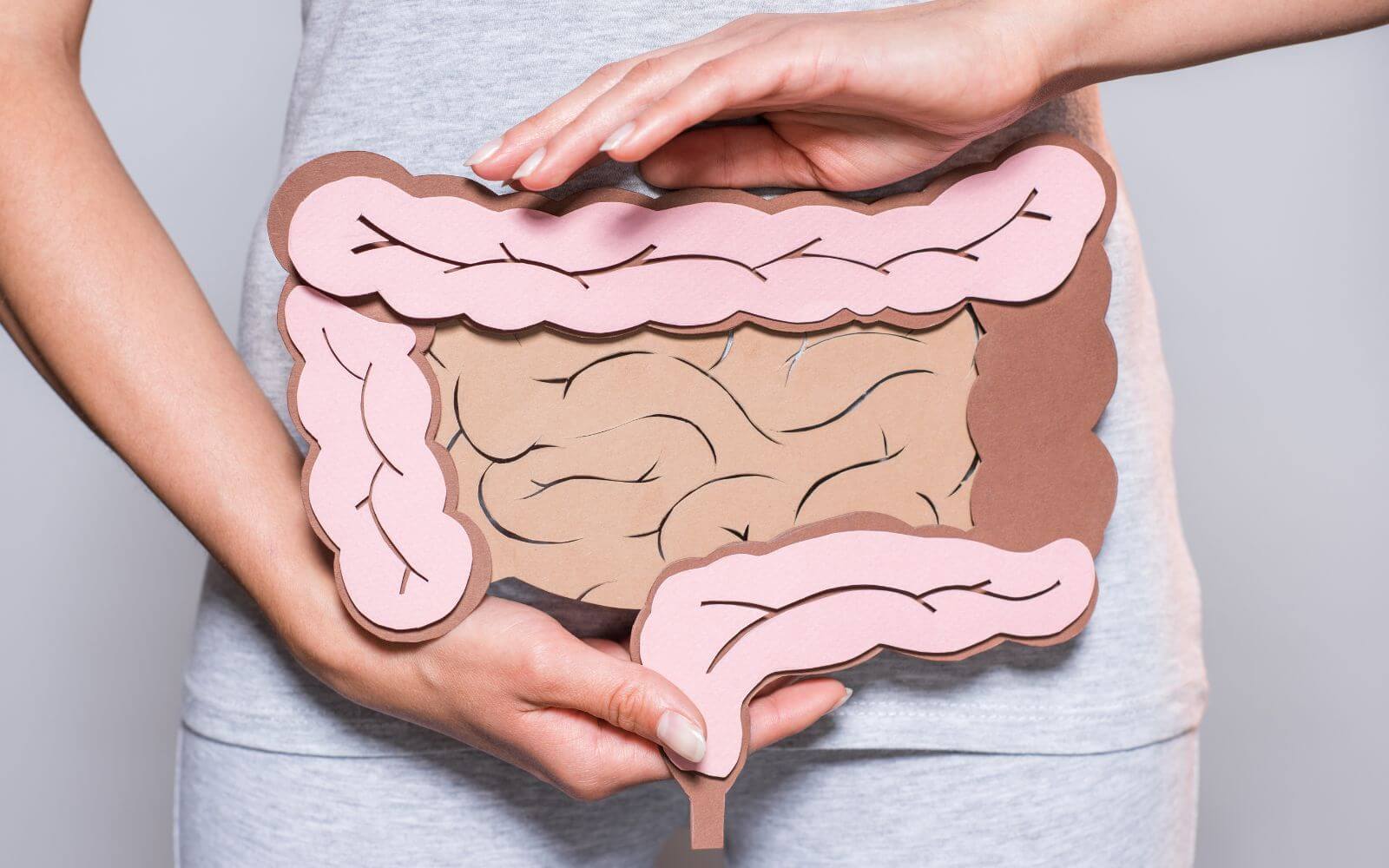
• X-ray contrast medium, especially for images of the gastrointestinal tract
Barium in nature:
Plants absorb barium through the soil and accumulate it. The highest barium concentration is found in Brazil nuts.
Ornamental algae (Desmidiaceae), which occur in cold, nutrient-poor freshwaters, depend on barium. Their cells show fluid-filled cavities containing small barium sulfate crystals. The exact function of these crystals has not yet been clarified, but the fact that barium is vital for plants is clearly shown by the fact that they simply cannot grow without it.
Barium is even found in the human body. We take in about one milligram of barium a day through our food.
Toxicity of barium
All barium compounds that are soluble in water or acid are toxic. For an adult human, a dose of 1 to 15 grams – depending on solubility – is fatal. Poisoning usually occurs in the workplace. Barium can be inhaled or enter the body through drinking water.
We would be happy to provide you with more detailed information:
- Barium acetate
- Barium bromide anhydrous
- Barium carbonate
- Barium fluoride
- Barium hydrogen orthophosphate
- Barium iodide dihydrate
- Barium nitrate
- Barium sulfate




 4c media
4c media